November 25, 2014
Hitachi Solutions, Ltd.
The National Center of Neurology and Psychiatry (NCNP, Kodaira, Tokyo; President: Teruhiko Higuchi) and Hitachi Solutions, Ltd. (Shinagawa-ku, Tokyo; Representative Director and President: Kaichiro Sakuma) have jointly developed the Remudy WEB Patient Information Registration System aimed at creating an online version of the muscular dystrophy patient information registration system "Remudy" that was launched by NCNP in 2009, and this will go into service from November 26.
This System has been constructed through applying NCNP's clinical research with the achievements of Remudy and the anonymous information management service "Tokumei Bank" (anonymous bank) of Hitachi Solutions. Through having patients themselves register disease information, including gene information online, and utilizing this as anonymous information, contribution can be made to developing remedies and innovative drugs for overcoming rare intractable diseases.
Moreover, by the end of March 2015, security for the System will be strengthened through applying the searchable encryption technology of Hitachi, Ltd.'s Yokohama Research Laboratory. In doing so, NCNP and Hitachi Solutions will provide a system by which numerous research institutes and medical institutions can equally utilize information.
For developing remedies and innovative drugs for overcoming rare diseases, it is necessary to have a mechanism that can rapidly bring together accurate epidemiological information and participants for clinical trials. NCNP established such a mechanism in 2009 when it launched the Registry of Muscular Dystrophy (Remudy).
Remudy WEB Patient Information Registration System allows patients to register and update information online. Moreover, registered patients can receive information on research and clinical trials in timely fashion through the system.
Introduction of this System, in collaboration with the Muscular Dystrophy Clinical Trial Network (MDCTN) started in 2012, will make a major contribution to the advancement of international remedy development and clinical research.
NCNP is taking steps to widely apply this System, developed through utilizing the know-how of Remudy, to other ailments, and it is anticipated that it will facilitate the furtherance of clinical trials, remedy development, and clarification of rare intractable diseases.
Hitachi Solutions from now on intends to apply system modularization of common functions and searchable encryption technology, and in doing so it will assist the cloud provision of the system and its application to other diseases.
Operation of a registration system for information of patients with rare diseases on a nationwide scale is the first initiative of its kind not only in Japan but also in Asia.
Remudy WEB Patient Information Registration System is the patient registry system that integrates Hitachi Solutions' "Tokumei Bank" with the searchable encryption technology of Hitachi, Ltd.'s Yokohama Research Laboratory. Searchable encryption technology enables only users (research institutes, etc.) who hold a key to search for encrypted information and decode the search results.
Thanks to this technology, it is possible to anonymously store personal information and clinical information on the cloud, and the provision of information management at a high-security and robust data center makes it possible to utilize information in accordance with ethical guidelines for clinical research.
Through standardizing the parts that are common irrespective of diseases, for example, the patient information registration function, screen design and so on, it has become possible to apply the technology to other disease registry systems in short time.
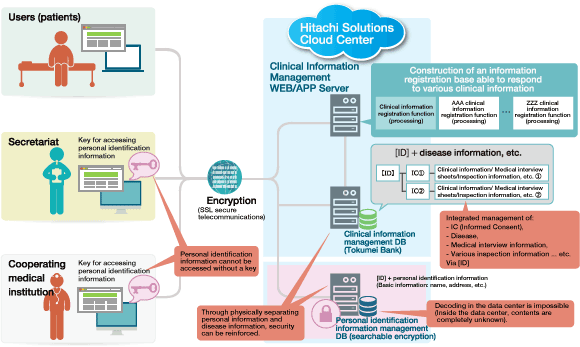
Figure: Mechanism of the Remudy WEB Patient Information Registration System
Utilization of Remudy
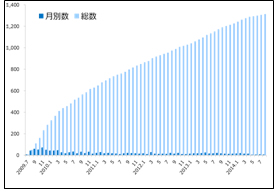
Table 1 Movements in the Number of Patients Registered to Remudy (Duchenne and Becker types muscular dystrophy)
Remudy is the information registration system for patients of dystrophinopathy (muscular dystrophy caused by dystrophin anomalies). As of October 2014, the number of registered patients is 1,322 patients diagnosed with the Duchenne and Becker types muscular dystrophy (Table 1) and 157 diagnosed with distal myopathy with rimmed vacuoles.
Many neuromuscular diseases are rare intractable diseases for which development of a fundamental remedy has been long awaited. NCNP utilizes the patient information of Remudy to conduct the following kinds of research.
1. Development of Nucleic Acid Drugs
Duchenne type muscular dystrophy is a disease that occurs when dystrophin protein, which protects the protective membrane around muscle tissue, degenerates as a result of gene mutation. In Japan, it occurs in the ratio of one case per 3,500 male infants and it is the most commonly occurring critical genetic disease, starting with muscle weakness and eventually leading to death. Antisense nucleic acid drug has been developed as a remedy for Duchenne type muscular dystrophy, and medical investigator-initiated clinical trials were started in July 2013. In addition to this, the patient information registered in Remudy is proving useful in international joint development activities in both Japan and overseas.
2. Joint Clinical Trials at Multiple Facilities by the Muscular Dystrophy Clinical Trial Network
The Muscular Dystrophy Clinical Trial Network, which entails conducting clinical trials and sharing information on a nationwide scale, was launched in February 2013, and joint medical investigator-initiated clinical trials at multiple facilities are being conducted under this. It is anticipated that the Remudy WEB Patient Information Registration System will be utilized to help promote the development of remedies for rare diseases in this Network too.
3. Substantiation of the Long-term Effects of Steroid Treatment for Muscular Dystrophy
In October 2013, NCNP conducted detailed analysis of data registered in Remudy, revealing that steroid treatment for Duchenne type muscular dystrophy extended the ambulatory period, and it published its findings in the Journal of Neurology. This was the first clinical research proving the long-term effect of a remedy for muscular dystrophy, and it was also the first time in the world that a patient registration system was used in research.
Responsible Department: Public Relations & Advertisements Group, Corporate Brand & Communications Department
Responsible Personnel: Ms. Ando
Tel: +81-3-5479-5013 Fax: +81-3-5780-6455
E-mail: koho@hitachi-solutions.com
* "Hybrid integration" is a registered trademark of Hitachi Solutions, Ltd.
* The names of other companies and products are the trademarks or registered trademarks of the respective companies.